AppGenex Diagnostics develops and commercializes multiplex diagnostics for the detection of infectious diseases
AppGenex Diagnostics develops and commercializes multiplex diagnostics for the detection of infectious diseases
AppGenex Diagnostics develops and commercializes multiplex diagnostics for the detection of infectious diseases
AppGenex Diagnostics develops and commercializes multiplex diagnostics for the detection of infectious diseases
AppGenex Diagnostics is a diagnostic company focused on the development and commercialization of multiplexed products for the diagnosis of infectious diseases, with an initial emphasis on tuberculosis (TB). Our technology is instrument platform agnostic in that we leverage the right instrumentation platform for the right diagnostic application that works for our target clinical market. Our current products and product candidates are based on bead-based flow cytometric multiplex technology. This technology has the ability to simultaneously detect multiple biomolecules, for instance, patient’s antibodies against specific Mycobacterium tuberculosis antigens, in a single sample. Importantly, the multiplex technology has the flexibility to be configured for high throughput and point-of-care needs for obtaining results in an economic and rapid fashion.
AppGenex Diagnostics has a pipeline of proteomic (antigen and antibody markers of disease) and molecular (nucleic acid) TB diagnostic products that address the key clinical decision points in diagnosis, treatment selection and treatment monitoring for TB detection and disease management.
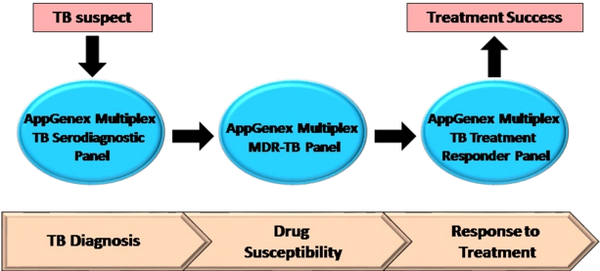
The AppGenex Multiplex TB Serodiagnostic Panel is our flagship product for the detection of active TB disease. This panel is a rapid, accurate and affordable blood-based TB diagnostic, intended for use in hospitals and clinics with high volumes of potential TB patients. This panel consists of eleven purified, recombinant pathogen antigens chemically cross-linked to fluorescently coded microbeads. The unique combination of antigens allows for consistent and best-in-class sensitivity and specificity for active TB disease detection. This test can be applied to patient samples consisting of a few drops of blood collected from a finger pinprick and processed directly from plasma or spotted on a filter paper disk and stored/transported for later reconstitution and testing. These simple sample collection options give this multiplex diagnostic test ease of use in almost any setting, particularly in countries where TB is endemic and the healthcare infrastructure is limited.
The AppGenex Multiplex MDR-TB Panel is a follow-on molecular diagnostic panel intended to be applied to patients who score positive in the Multiplex TB Serodiagnostic Panel. This panel detects multi-drug resistant TB (MDR-TB) and will aid in the selection of the proper antibiotics for treatment.
The AppGenex Multiplex TB Treatment Responder Panel follows key biomarkers in the blood of TB patients that correlate with antibiotic treatment success. Knowledge of treatment efficacy can help track patient compliance and enable treatment management to mitigate the emergence of MDR-TB.
Professor
Department of Pathology and Laboratory Medicine
School of Medicine
University of California, Davis
https://www.linkedin.com/in/imran-khan-a4b38a2
Professor Emeritus
Department of Pathology and Laboratory Medicine
School of Medicine
University of California, Davis
https://www.linkedin.com/in/paul-luciw-6556343
Mountain View, CA